Description
Product description
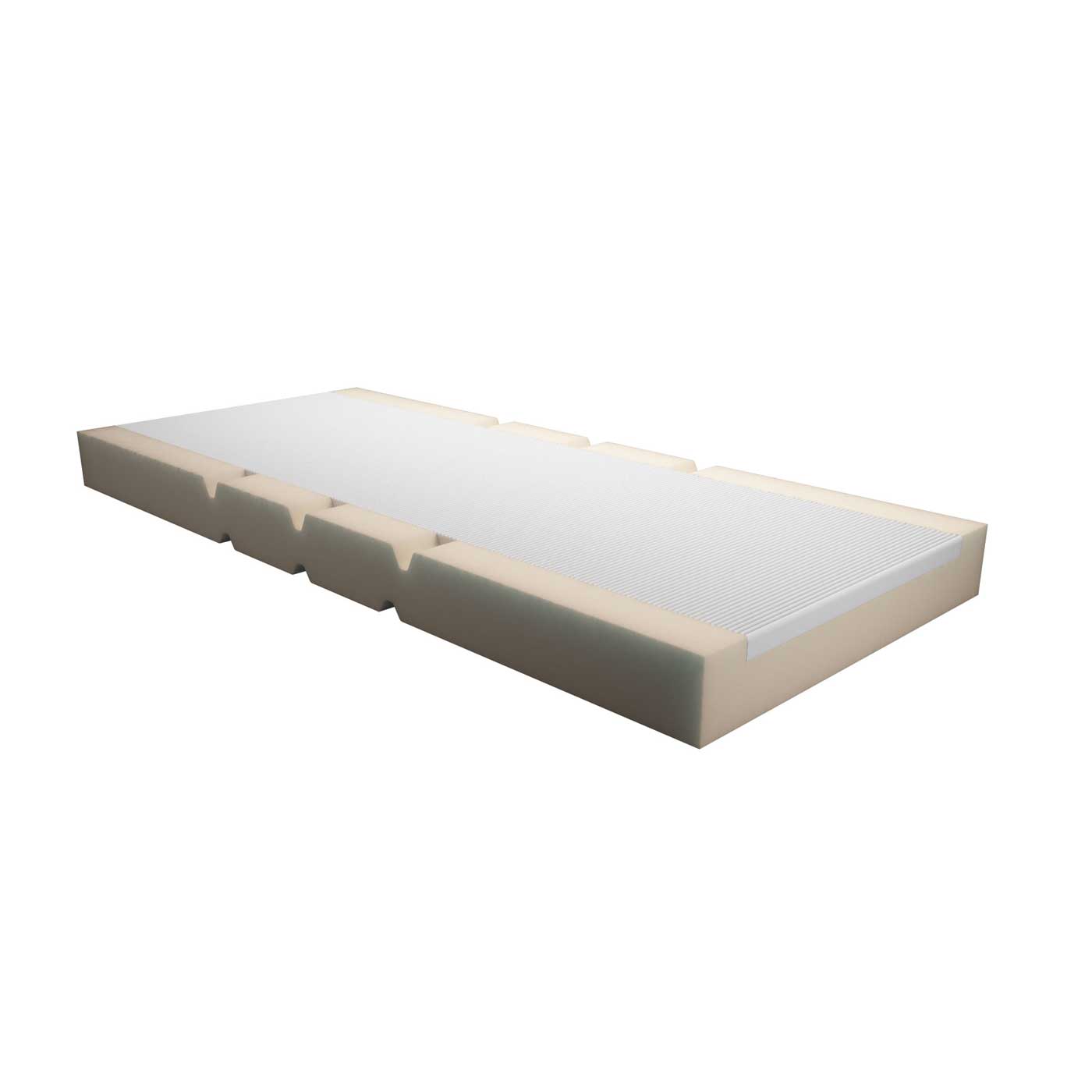
Static bimodular mattress made of high-performance open-cell polyurethane foam with thermo-pressurised support surface made of 50S-VE (viscoelastic technopolymer – slow memory). For general, specialist and low to medium decubitus risk hospitalisation, including paediatric and neonatal.
The mattress is equipped with a special waterproof, breathable, bi-elastic, antistatic, self-extinguishing cover treated with the Zeneca bacteriostatic-fungostatic system. Indicated for a posture with a very low compressive gradient on the muscular-skeletal apparatus functional for the prevention of pressure skin lesions and painful and inflammatory symptoms, useful in incontinent patients and for better epidemiological control.
CE marked medical device, performance requirements certified UNI 10707 rev. 2003, comfort and anti-decubitus index certification (ergocert D28) and approved in fire reaction class 1IM.
Functional capacity minimum Kg 0 maximum Kg 180.
Sag factor – elastic polyurethane comfort index – not applicable.
Blue colour.
Hypoallergenic, non-toxic, odourless and silent product.
Product that allows cardiac massage.
Latex Free product.
Radiotranslucent product.
Basic density 30 kg/m2 and average bearing capacity 3.3 Kpa – memory foam support
(Other specifications and densities available on request)
by Synergic Italiana


 Italiano
Italiano  Français
Français  Deutsch
Deutsch  Español
Español  Nederlands
Nederlands  polski
polski  Norsk bokmål
Norsk bokmål  Magyar
Magyar  Română
Română  Dansk
Dansk